Pure aluminum is clearly better than Zinc Nickel Plating
Electroplated aluminum is a superior choice to low hydrogen embrittlement zinc nickel (LHE ZnNi) plating, offering more uniform composition, improved embrittlement mitigation, and unsurpassed corrosion performance and fluid compatibility.
Pure electroplated aluminum and LHE ZnNi are the leading commercial alternatives to cadmium for protection of aerospace components. Extensive DoD OEM testing indicates AlumiPlate® aluminum consistently outperforms LHE ZnNi and has become the premier anti-corrosion coating for high strength components in severe environments.
Typically More Than 99.99% Pure Aluminum Has Unsurpassed Performance in Sulfur Dioxide (SO2)
Aluminum plating benefits from the synergistic effects of purity, a dense structure, high electronegativity, chemical resistance and a self-healing protective oxide layer. The result is unsurpassed barrier and sacrificial corrosion resistance imparted to nearly any base material.
Accelerated corrosion testing has been used historically to determine the relative corrosion resistance of coatings like ASTM B-117 salt fog testing. With the higher performance envelope of new aircraft, more stringent types of accelerated corrosion testing using sulfur dioxide (SO2) are the current barometer for coating performance in severe environments.
Electroplated aluminum leads the way in accelerated and environmental corrosion protection.
In side-by-side testing with ZnNi and a Cd control, aluminum plated 4130 steel coupons showed extremely high corrosion resistance. To test both barrier and galvanic (sacrificial) protective capabilities, the coatings were scribed prior to ASTM G-85 cyclic SO2 salt spray testing. Plating thickness was equivalent at 12.5 micrometers minimum.
Specimens with zinc nickel plating showed indications of red rust after 168 hours, while electroplated aluminum specimens lasted 668 hours before indications of base metal corrosion (refer to photos below in Figure 1). The impressive performance of scribed coupons with exposed steel is a testament to the extraordinary sacrificial protection capabilities of electroplated aluminum.
Electroplated aluminum helps achieve maximum protection and extends the life of components in a severe environment. With over 20 years of experience, AlumiPlate can engineer and help specify a custom solution based on your application needs.
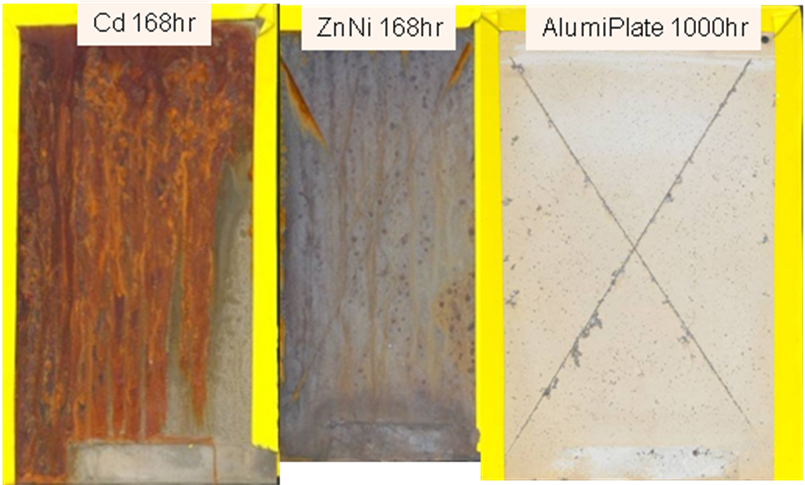
Figure 1: IAW ASTM G-85 Annex 4 Modified Cyclic Sulfur Dioxide SO2 Accelerated Corrosion of 4130 Steel Coupons (Coated and Scribed).
Electroplated Aluminum VS. ZnNi Properties and Benefits
High purity electroplated aluminum offers a distinct set of properties and benefits that cannot be matched by ZnNi. Some of the advantages and features are listed below in tabular form.
| Specs | AlumiPlate® AL | LHE ZnNi |
|---|---|---|
| Nominal Recommended Thickness | 0.0003" | 0.0003" |
| SO2 (ASTM G-85) Performance | 668 hrs | 168 hrs |
| Salt Spray (B-117) Performance | 1000+ hrs | 1000+ hrs |
| RoHS and REACH Compliant | Yes | Yes (Now), Future(?) |
| Uniform Composition and Purity | Yes, 99.99% Al | No (5-10% Ni) |
| No HE, No Re-Embrittlement & No 24-Hr HE Bake | Yes | No |
| High Temperature Capability | Up to 1000 °F | Up to 1000 °F |
| Sacrificial Protection | Yes | Yes, but lower |
| No Galvanic Reaction with SST, Ti, BeCu, AlNiBr | Yes | No, in salt water |
| Process Not Susceptible to Organic Contamination | Yes | No |
| Bath Agitation Unrelated to Coating Embrittlement | Yes | No |
| No Anode Degradation Risk or Coating Impact | Yes | No |
| Ductile, Formable and Stampable | Yes | No |
| Anodizeable | Yes | No |
Table 1: Properties and benefits of electroplated aluminum versus ZnNi.
Aluminum Plating Benefits from Uniform Composition and a Stable Process
Electroplated aluminum is typically greater than 99.99% pure, providing consistent properties and predictable performance for all part geometries and operating environments. The AlumiPlate® aluminum electroplating process is highly automated, controlled and monitored. The process chemistry is continuously filtered and controlled within +/- 1 °C. The electrodeposition process precludes contamination of the coating. Trace metals are insoluble and filtrated in-process; organic contaminants are dissolved and eliminated in pre-treatment baths.
LHE ZnNi can have variable composition with a content ranging 5-15% Ni. Performance and properties cannot be expected to match the very tight and consistent distribution of “4-nines-pure-aluminum.” Furthermore, the zinc nickel plating processes are sensitive to temperature variations, agitation, organic contamination and anode degradation. Zinc anodes can degrade exothermically, releasing heat which negatively affects ZnNi composition. More disturbingly, the ZnNi process is extremely susceptible to organic materials. Any organics present in the bath, and even in plumbing lines, will negatively affect the quality of the ZnNi coating. Finally, bath agitation cannot be used during ZnNi plating because it averts potential embrittlement of high strength steels, further increasing the variability of the process and coating.
Electroplated Aluminum Mitigates Hydrogen Embrittlement and Re-embrittlement
The AlumiPlate® aluminum electrolyte is aprotic and completely free of hydrogen cations (H+). Without a source, substrate absorption of hydrogen (hydrogen embrittlement) is precluded during the plating step.
In the field, coated high strength steels are susceptible to re-embrittlement. This phenomenon involves generation of hydrogen during the corrosion process, while the sacrificial coating galvanically protects the substrate. Hydrogen is then free to migrate into the base metal, reducing its toughness and potential uncontrolled brittle failures at much lower than expected loads.
In numerous embrittlement and re-embrittlement experiments, aluminum plating has shown an exceptional and unmatched capability to mitigate both embrittlement and field re-embrittlement.
Side-by-side testing with coated ZnNi (ASTM F-519 and ASTM F-1624) confirms that pure aluminum prevents embrittlement and re-embrittlement of high strength 4340 steel specimens. More importantly, plated aluminum specimens did not exhibit re-embrittlement, unlike ZnNi specimens. Embrittlement caused during plating may be addressed with a relief heat treatment (the typical 24-hour HE bake required of Cd, Ni and ZnNi). However, field re-embrittlement (environmentally induced cracking or, when under tension, stress corrosion cracking) is far more insidious and cannot be alleviated. The best solution to combat embrittlement and re-embrittlement mechanisms is electroplated aluminum.
In ASTM F-1624 re-embrittlement testing, 4340 steel specimens were coated with ZnNi and tested under load while immersed in seawater (ASTM D1141) and reagent water (ASTM D1193). After the initial 24-hour load to 45% notched fracture strength (NFS), the load was incrementally stepped by 5% NFS until failure.
Results for ZnNi were poor with an average NFS of 58% in seawater and 81.6% for reagent water. Some ZnNi specimens immersed in seawater were alarmingly noted to have failed during the initial load phase at only 45% NFS.
Electroplated aluminum significantly outperformed zinc alloy plating with an average NFS of 96.45% in seawater, 95.35% in reagent water and zero early failures. The test results confirm that aluminum plating is the best sacrificial coating option for high strength components that operate in fluid environments.
Please refer to our hydrogen embrittlement page for more information.
An AL4N Coating AL Alloys With STT, TI, HSLA Steels and BECU
Improved Galvanic Protection
Aluminum alloys are ubiquitous in aerospace and other industries requiring strong and lightweight materials. Steel and other high strength alloys like Al6V4Ti are used complementary for structural support or critical applications that require a higher strength than an Al alloy can provide.
Finished assemblies bring many different metals in contact with aluminum alloys, from beryllium copper (BeCu) to aluminum nickel bronze (AlNiBr). There is potential for dissimilar metal corrosion in the field due to the high electronegativity of aluminum alloys.
When tested in salt fog and cyclic SO2 chambers, aluminum plating stopped galvanic corrosion. The assemblies consisted of Al 2024 and Al 7075 test blocks, stainless steel (SST) bolts, and a variety of coated washers (4130 Steel, 17-4 SST, BeCu and AlNiBr). The change in resistivity of the coated articles (washer under a torqued bolt) was measured after accelerated corrosion.
Electroplated aluminum articles showed little or no increase in resistivity after salt fog testing. ZnNi washers had much higher resistivity, especially in the Al 7075 test assembly. Both coatings showed little change in resistivity in cyclic SO2 testing. However, several ZnNi articles were noted to have broken under load during corrosion testing.
Electroplated aluminum is the ideal choice for eliminating galvanic or dissimilar metal corrosion.
Pure Aluminum Shows Augmented Compatibility with Aerospace Fluids
Due to its high purity, density, corrosion resistance and uniformity, electroplated aluminum is compatible with a wide range of aerospace fluids. Compatibility was confirmed via immersion testing in specific fluids for 7 days at 100 °F +/- 2 °F followed by a 16-hour desiccation period.
When immersed in reagent water, synthetic sea water, aircraft and runway de-icing fluids, parts washer, aerospace cleaning compound, paint removers, wheel well cleaner, hydraulic fluids and propylene glycol in DI water, the aluminum plating protects 4340 steel with no significant surface reaction.
This performance cannot be matched by ZnNi plating, which reacts after immersion with many aerospace fluids and is especially sensitive to paint removers and hydraulic fluids.
Aluminum plated aerospace components can be expected to last longer and are applicable to more service environments than any other coating.
AlumiPlate Aluminum is 100% RoHS, REACH and Future-Proof Compliant
AlumiPlate aluminum is typically greater than 99.99% pure and meets RoHS and REACH guidelines (please see our environment page). Aluminum plating is one of the few metal coatings that is not targeted by future regulations.Both international and domestic regulatory groups are contemplating further restrictions on nickel, zinc, cadmium, lead and tin.
Per a May 2017 report from the National Association for Surface Finishing (NASF), California’s South Coast Air Quality Management District (AQMD) wants to restrict levels of nickel in the air to less than 0.2 nanograms per cubic meter, which is the same control as toxic hexavalent chromium.
The NASF report states that compliance for ZnNi surface finishers will be extremely expensive and challenging since the AQMD regulation will target fugitive plating emissions. The implication is that the cost of ZnNi will increase dramatically, since few surface finishers will be financially and technically capable of installing and monitoring abatement equipment. Many finishers may actually cease offering zinc nickel plating services due to the growing expense and liability.
Electroplated aluminum is non-toxic, recyclable and deposited using enclosed equipment with potential for zero emissions. It complies with present regulations and offers worry-free and future-proof compliance. Including aluminum plating in your design is beneficial to the environment and will minimize future compliance issues.
Contact us by phone call or email for more information on how we can assist you with your application in greater detail.